Who we are

At APLUSA, we turn deep expertise, innovation, and a deep understanding of the needs of our clients, healthcare professionals and patients into insights that enable high quality decisions.
I am inspired by our teams as they bring scientific rigor and creativity to simplify complexity, deliver precise and meaningful recommendations, while consistently exceeding expectations.
I am proud of the way we build real partnerships with our clients, grounded in trust, active listening, and collaboration. Our goal is to create real impact across highly complex and technical therapeutic areas such as hematology, oncology, immunology, and dermo-cosmetics.
Innovation, the cornerstone of our strategy, drives APLUSA ability to anticipate market shifts, pioneer new methodologies, and design tools that empower our clients in their daily work. What makes this innovation truly effective, in my view, is our people: agile, open, and committed teams working together to generate lasting value and genuine impact.
Gema Parlange Iturmendi,
CEO
Who we are

APLUSA is one of the world’s leading independent market research companies dedicated to healthcare.
Thanks to the excellence and commitment of our teams, our challenge for globalization has been well and truly met: more than half of our turnover is generated overseas, two-thirds of our services are international (conducted in 75 countries), and our teams are a true reflection of our business: multi-nationals. We are now, globally, among the Top 10 Global Healthcare Market Research Companies, and the Top 3 Independent Players.
We are proud to have achieved ISO 20252 certification, which represents more than 30 years of passionate commitment together with our clients – both healthcare companies and institutions – working through them in the service of healthcare professionals and patients around the world.
The decisions that our clients make can have a significant impact on the health and well-being of populations around the world; the quality of our data, the relevance of our analyses, and the reliability of our recommendations make us an integral part of the process.
The convergence of the spheres of healthcare and information technology opens a host of new opportunities; together, we will work to provide the very best tools to our customers to support their initiatives, inform their decisions, and validate their strategic planning. Acquiring new knowledge; developing, deploying, and improving our know-how; innovating; anticipating and supporting regulatory change: these are the bases of excellence and the commitment of our teams.
The ISO certification process was essential for us to evaluate – measured against the most demanding standards – our ability to develop and execute our products, services, processes, and quality management systems to the expectations of our clients and the aspirations of our employees. “Being ISO” means committing to permanent and continuous improvement with an eye toward building the company of the future and being a privileged partner to our clients.
Strengthened by our expertise, our stable and committed shareholder base is there to support our strong growth. Our ambition is to build an exquisite enterprise, of great worth and shared values.
We take responsibility regarding our ecosystems and sociosystems, carrying out our mission by acquiring new skills and attracting new talents, developing new products and solutions, establishing partnerships, furthering our business models, and advancing our associates. For the men and women of APLUSA, those who are with us today, as well as those who will be joining us tomorrow, we will provide a safe space to foster their talents and creativity. Such is the condition and the measure of our success.
Gema Parlange Iturmendi,
CEO
Our mission
Our job is to help healthcare manufacturers and practitioners optimize their decisions by supporting them with reliable insights.
To do this, we collect healthcare data around the world and transform them using our technologies and our talents to give our customers strategic and operational guidance in order to improve the reliability of their decisions.
We take responsibility regarding our ecosystems and sociosystems, carrying out this mission by offering our employees the opportunity to flourish in meaningful and rewarding work.

Our DNA
Our DNA: Professional elegance
Achieving simplicity and clarity in our approach and in the solutions we offer.

Our VALUES

Strive for excellence
Our integrity, diligence and flexibility underpin our commitment to offering the very best service to our clients.
These principles drive our behaviour and the choices we make.

Fuel exploration through our diversity
We leverage the richness of our diverse backgrounds to continuously explore, improve our knowledge, and innovate to meet emerging healthcare challenges.

Grow and thrive
We work with integrity, commitment, passion, and generosity of spirit, creating an environment where every individual can express themselves and develop their talents to the highest potential.

Nurture our shared ambition
As a team, our actions are guided by respect and responsibility, recognizing that together, we can achieve far more and with far better results than even the most talented of us could achieve alone.
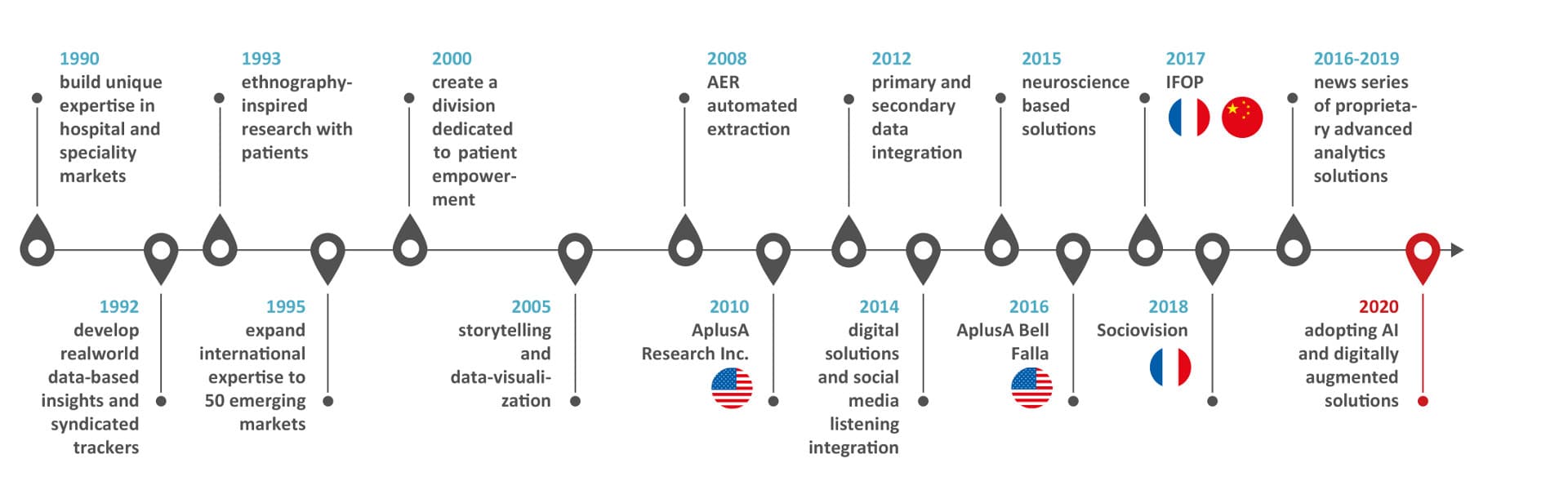
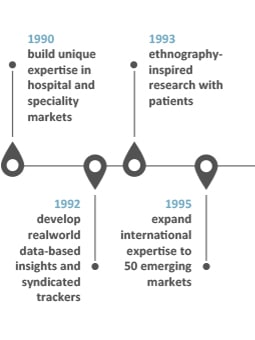
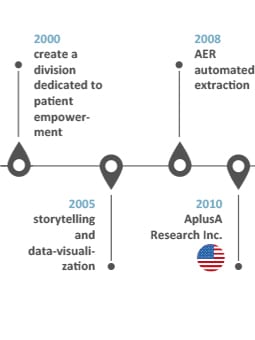
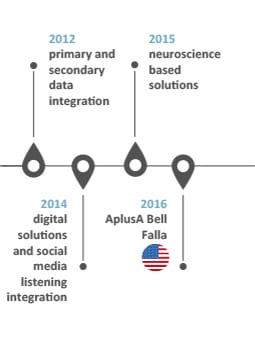
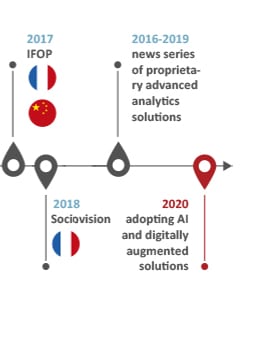
Our history of innovations
INNOVATION: “Something different that has impact” Scott D. Anthony – HBR
Our history speaks for our capacity to think forward and welcome successful companies
into our organization to embrace impactful innovative solutions for our clients:
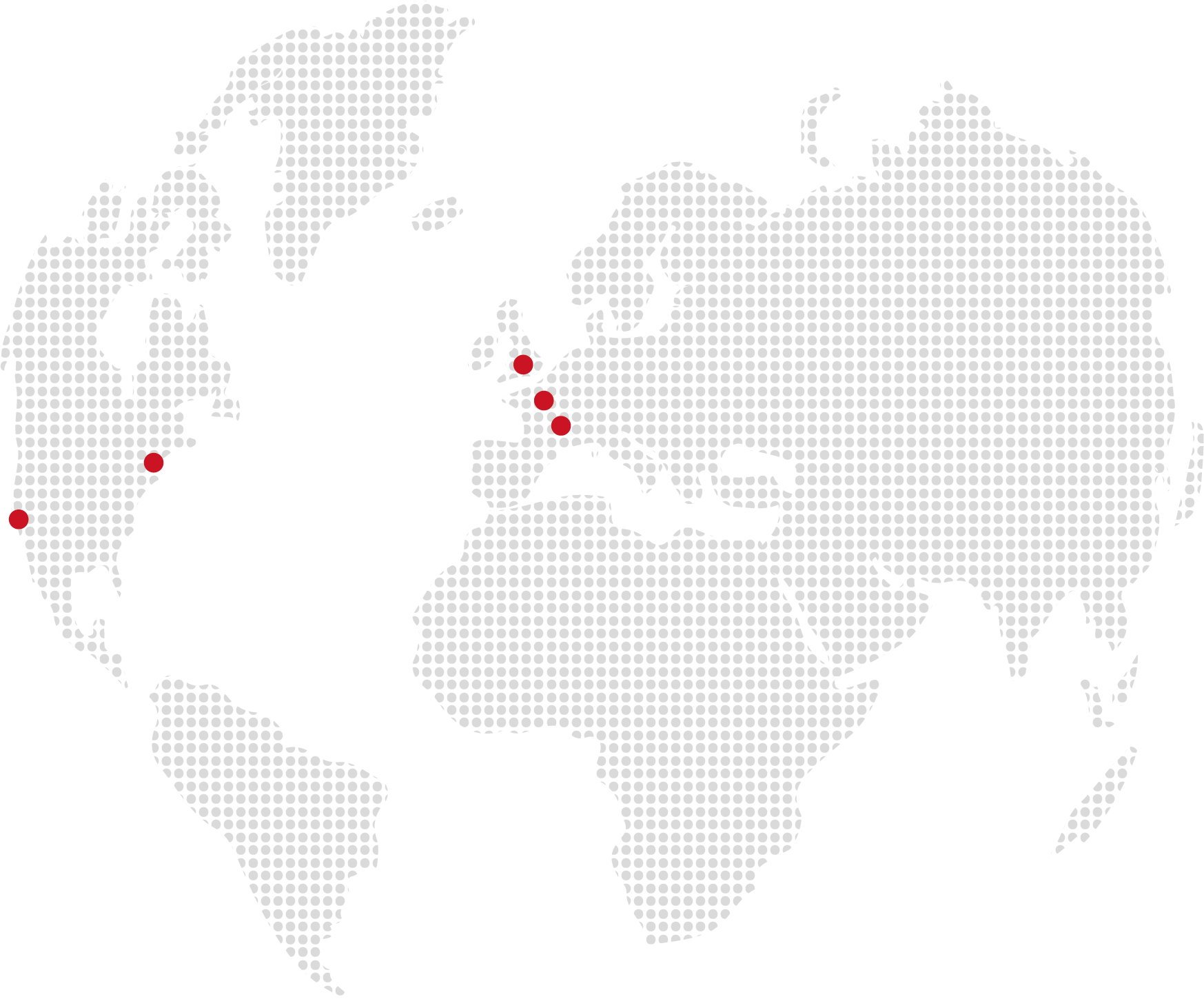
Our global footprint
WE ARE TRULY GLOBAL:
We are multicultural by nature with over 30 nationalities represented in our teams including people from Benin, Cameroon, Canada, Finland, France, Germany, Hong Kong, Italy, Ivory Coast, Lithuania, Mauritania, Mexico, Morocco, Nepal, Peru, Philippines, Poland, Portugal, Romania, Russia, Senegal, Singapore, Somalia, Trinity, Tunisia, Turkey, UK, Uruguay, USA, Vietnam, …
Our areas of expertise
APLUSA is founded and led by physicians, so clinical knowledge is at the core of everything we do. We have over 30 years of experience in conducting healthcare market research studies across all major therapeutic areas, including rare diseases, that answer macro and micro market questions. Our highly experienced teams combine unparalleled healthcare market understanding with augmented solutions in order to deliver in-depth and actionable information to our clients.
Our expertise focuses on three main areas: unparalleled patient charts expertise, long-established performance trackers, and an ever-evolving range of syndicated solutions, augmented with insights from advanced analytics.
Our organization
APLUSA‘s organization mirrors our client’s organization and reflects our ambition to guide and monitor the healthcare industry transformation
Our organization, balanced between the US and EU, with a completely integrated management, matches our client’s organization and ensures senior attention and consistency across regions
APLUSA teams
Leadership team
Passionate about building exceptional teams and developing innovative solutions, Gema joins APLUSA as CEO in December 2024.
With 30 years of experience in the life sciences, she has held key roles in finance, sales, and general management at leading organizations such as IQVIA and Parexel. Gema was also a co-founder of Lumanity, where she served as Chief Development Officer and Global Head of Real-World Evidence (RWE).
A Spanish citizen, she has lived in Germany, Switzerland, and the UK, and currently splits her time between Madrid, Lyon, and wherever else her customers or team need her. Gema is married, has two children, and holds a double degree in Economics and Business Administration from the University of Deusto.
Gema Parlange Iturmendi
Chief Executive Officer
Christine joined AplusA in 1990 and supports the senior business directors to develop client relationships worldwide. She is responsible for identifying new partnerships and developing innovative solutions for AplusA clients. Christine is a qualified MD specializing in palliative care and holds an MBA in Pharmaceutical Marketing.
Christine Maï, MD
Deputy Managing Director
Chief Sales & Marketing Officer
Driven by a constant desire to connect finance with operational efficiency, with over 16 years of experience in finance and financial control, Cyrille joined APLUSA as Chief Financial Officer in September 2025. He is responsible for implementing financial strategy and financial performance to support business growth and transformation. Convinced that financial culture is a key factor for success, Cyrille is committed to fostering a strong financial culture so that it becomes an essential part of decision-making, giving APLUSA everything it needs to achieve its ambitious goals.
Cyrille Plaza
Chief Financial Officer
After a 20 year successful career in various human resources positions, Sophie Benes joins AplusA as our Human Resources Director. She provides our team with a French American experience gained in different Silicon Valley start-ups and other HighTec and industrial companies in Rhône-Alpes.
She is in charge of developing and implementing talent management through individual and collective coaching in our multi-cultural and multi-national environment.
Sophie Benes
Human Resources Director
Passionate about Data and AI, and with more than 20 years of experience in technologies, Laurent joined APLUSA as Chief Data Officer in July 2025. He is responsible for designing and implementing a robust data strategy that drives transparency, productivity, revenue growth and business transformation. Strong advocate of Artificial Intelligence as a key driver of innovation and market acceleration, Laurent focuses on building a solid AI culture, empowering teams with AI expertise, implementing effective governance frameworks, and leveraging AI to enhance APLUSA’s solutions.
Laurent Givaudan
Chief Data Officer
Pierre founded APLUSA, formerly ISIS Research in 1989. Pierre is Shareholder of APLUSA and Board Member. He is a qualified MD specializing in Endocrinology and Nutritional and Metabolic disorders.
Pierre Pigeon, MD
Founder, Board Member
APLUSA teams
Leadership team
Passionate about building exceptional teams and developing innovative solutions, Gema joins APLUSA as CEO in December 2024.
With 30 years of experience in the life sciences, she has held key roles in finance, sales, and general management at leading organizations such as IQVIA and Parexel. Gema was also a co-founder of Lumanity, where she served as Chief Development Officer and Global Head of Real-World Evidence (RWE).
A Spanish citizen, she has lived in Germany, Switzerland, and the UK, and currently splits her time between Madrid, Lyon, and wherever else her customers or team need her. Gema is married, has two children, and holds a double degree in Economics and Business Administration from the University of Deusto.
Gema Parlange Iturmendi
Chief Executive Officer
Christine joined AplusA in 1990 and supports the senior business directors to develop client relationships worldwide. She is responsible for identifying new partnerships and developing innovative solutions for AplusA clients. Christine is a qualified MD specializing in palliative care and holds an MBA in Pharmaceutical Marketing.
Christine Maï, MD
Deputy Managing Director
Chief Sales & Marketing Officer
Clément is our Chief Financial Officer. His previous engagements as CFO in the international consulting and professional services industry for high-growth companies makes him a valuable asset for APLUSA in driving our growth. For him, finance is at the service of strategy and operational staff who innovate, sell and produce. Finance must enable them to work in a serene climate, based on reliability, consistency and honesty – values that are perfectly aligned with APLUSA’s mission and principles. Clément holds a Master’s Degree in Management and Finance from EDHEC Business School.
Clément Camozzi
Chief Financial Officer
After a 20 year successful career in various human resources positions, Sophie Benes joins AplusA as our Human Resources Director. She provides our team with a French American experience gained in different Silicon Valley start-ups and other HighTec and industrial companies in Rhône-Alpes.
She is in charge of developing and implementing talent management through individual and collective coaching in our multi-cultural and multi-national environment.
Sophie Benes
Human Resources Director
Passionate about Data and AI, and with more than 20 years of experience in technologies, Laurent joined APLUSA as Chief Data Officer in July 2025. He is responsible for designing and implementing a robust data strategy that drives transparency, productivity, revenue growth and business transformation. Strong advocate of Artificial Intelligence as a key driver of innovation and market acceleration, Laurent focuses on building a solid AI culture, empowering teams with AI expertise, implementing effective governance frameworks, and leveraging AI to enhance APLUSA’s solutions.
Laurent Givaudan
Chief Data Officer
Pierre founded APLUSA, formerly ISIS Research in 1989. Pierre is Shareholder of APLUSA and Board Member. He is a qualified MD specializing in Endocrinology and Nutritional and Metabolic disorders.
Pierre Pigeon, MD
Founder, Board Member
Business Development and business unit Directors
Eric has joined APLUSA after over 20 years dedicated at growing exceptional relationships with clients in the pharmaceutical industry across Europe and Asia. Eric brings in a solid expertise in the sale of services (market research studies, databases, panels of physicians and pharmacies) to help implementing, together with our Business Unit Directors and experts, commercial partnerships that will match even more our clients’ expectations. Graduate of the Ecole Supérieure de Gestion (now Paris School of Business), Eric also holds a master’s degree in economic and social administration from the University of Paris-Assas and an Executive MBA in healthcare management from the University of Paris-Dauphine.
Eric Yonter
Business Development Director
Elen joined APLUSA in 2005 and has over 15 years of experience in Healthcare Market research. Moving from Project Manager to Research Director positions, she conducted qualitative and quantitative research, with more focus on respiratory diseases, rheumatology, ophthalmology, dermatology, neurology, diabetes, medical devices, and Women’s health. Elen holds a Master degree in Marketing and Business management performed in France, Canada and the UK.
Elen Treuveur
France Business Unit Director
Fabrice has over 15 years of experience in healthcare Market Research. His first position was at APLUSA in Lyon from 2005 to 2008 before holding various senior positions in other international healthcare market research companies in Switzerland, the UK, and the US where he has settled permanently, before returning to APLUSA in 2023. Fabrice is passionate about innovative research methodologies uncovering human truths to inform brand development strategies. Fabrice also loves transforming insights into clear, simple, and actionable business solutions. Fabrice is a specialist in several therapy areas, in particular Immunology and HIV. Fabrice holds a Master’s degree in chemical process engineering, followed by an MBA in Pharmaceutical Marketing and Management from ESCP Business School.
Fabrice Dussol
US Business Unit Director
With 15 years of experience in market research and strategic marketing (mainly at Publicis Healthcare), Geoffroy Cornette has joined forces with the team at AplusA to accompany clients from initial consultation to project completion, providing critical strategic planning support. Geoffroy uses his expertise and passion in prescription markets to help you to map patient journeys, uncover prescription drivers, optimize communication, …, with a focus in oncology, hematology, virology, respiratory disease, and dermatology. Geoffroy graduated from EDC Paris Business School and IDRAC Business School.
Geoffroy Cornette
France Business Unit Manager
Hilary has over 25 years of experience in healthcare, having begun her career in the pharma industry before moving agency-side. She specializes in international/global studies, both qualitative and quantitative, and is passionate about the application of market research insights to support business decision making, as well as advocating for the voice of the patient in market research. Hilary is a specialist in several therapy areas, in particular multiple sclerosis and oncology, and has a literature degree from Leicester University as well as postgraduate marketing qualifications.
Hilary Worton
International Business Unit Director
Justin started his career at APLUSA in 2014 as a Market Research Executive, specializing in prescription analysis and ATU studies. Elevating swiftly, he assumed a leadership role as a Project Manager in the French Quant Team, overseeing comprehensive quantitative research across various stages of the product lifecycle. In 2021, Justin transitioned to the international team, where he currently serves as Business Unit Manager, showcasing profound expertise in Hematology (DLBCL, CLL, ALL, Multiple Myeloma), Oncology (Breast, Lung, Ovarian, Prostate), and Immunology. Justin holds a master’s degree in Marketing and Strategy.
Justin François
International Business Unit Manager
After first working in consumer research, Magali joined APLUSA in 2002 and specializes in patient chart studies covering all stages of the product lifecycle.
Through her expertise in a wide range of disease areas (hematology, oncology, infectious diseases, dermatology, CNS). Magali graduated in Psychology and holds a Master’s degree in Market Research, Marketing and Communication (Sciences Po Grenoble).
Magali Marion
France Business Unit Director, Prescription
Manuel has over 17 years of experience in the pharmaceutical and MedTech market research industry. He began his career at APLUSA where he went through different positions until research director, managing large international projects during 10 years in different disease areas including oncology, hematology, cardiology, respiratory, diabetes, ophthalmology, aesthetics, infectious diseases, etc. In 2015 he joined bioMérieux as Global Market Research Manager where he supported marketing teams over 7 years to collect the voice of the customer to inform decision making for their major brands in the fields of microbiology, molecular biology and immunoassay. He also provided support in other strategic projects related to the evaluation of new portfolio opportunities. Manuel joined back APLUSA as Business Unit Director. Manuel graduated in Business Administration in the University of Granada (Spain) and complemented it with a master’s in international trade and European affairs from IAE Lyon.
Manuel Guzman Martin
International Business Unit Director
Specializing in French domestic market research studies, Marie-Christine Ducrot serves as a Business Unit Director. She has a particular interest in market research involving psychological aspects of physicians’ decisions including relationships between patients/physicians and physicians/pharma companies as well as the evolution of the role of HCPs and the global impact. Marie Christine holds a DEA in Experimental and Social Psychology
Marie Christine Ducrot
France Business Unit Director
Mélanie joined APLUSA in 2009. She has developed tremendous expertise in prescription research over the years, holding the role of Director of the production team, bringing in her specific skills of past scientific researcher in CNRS (the French National Centre for Scientific Research, among the world’s leading research institutions). She is now helping you to design and conduct any type of market research based on patient charts, with experience in numerous domains such as oncology, immune-oncology, rheumatology, dermatology, gastro-enterology, hematology, ophthalmology, cardiology, etc. Mélanie holds a PhD in Biology and Chemicals.
Mélanie Marrot, PhD
France Business Unit Director, Prescription
Nadège joined APLUSA in 2020 to develop international market research in OTC medicines and products.
She has a strong background in marketing and product management (conditions: anxiety, stress, baby teething, naupathia… / products: dietary supplements, medicines, cosmetics, medical devices). Nadège started her career agency side as a quantitative market research executive before working client side in OTC products for a pharmaceutical company.
Her roles have included data research executive, then international product manager and head of market research and data panels. Nadège is a marketing graduate (Toulouse Business School), she speaks Italian, French and English.
Nadège Meillier
International Business Unit Director OTC & Consumer Healthcare
Pascale has over 20 years of experience in Healthcare market research and joined APLUSA in 2006. Psycho-sociologist by training, Pascale has developed expertise in qualitative and quantitative techniques and is particularly interested in research looking at all environment around patient with chronic or rare diseases (patient journey, interactions with caregivers and HCPs). She is also expert in OTC and consumer healthcare, nutrition, skin care, pediatrics, and rheumatology. Pascale holds a Master degree in Psychology and Sociology with specialization in consulting.
Pascale Bausson
France Business Unit Manager
Paul has been working in international healthcare market research for over 20 years, and is based in our London office. Paul is responsible for supporting global and UK-based clients with their needs across all therapy areas and methodologies. Paul enjoys studies that include patients, as well as those relating to medical devices, and spent numerous years working with longitudinal data from hospital records in Europe. Paul has previously worked with Aequus Research, Medimix and IQVIA, successfully developing new business among pharmaceutical and Biotech clients in Europe, Japan, Latin America and the USA. Paul has a wealth of experience within various therapeutic classes, noticeably in Oncology and Multiple Sclerosis as well as Diabetes, Epilepsy, HIV, pain management and Anti-Infectives.
Paul Taylor
International Business Unit Director
Rose-Marie Carneiro is a Business Unit Director with more than 20 years’ experience in pharmaceutical market research in both France and Australia.
As an expert in both qualitative and quantitative market research techniques, Rose-Marie pays special attention to patients and consumers in different therapeutic areas such as CV, respiratory, CNS, diabetes, aesthetics and cosmetics, women’s health, baby nutrition, senior nutrition, pain management and wellbeing. On top of this, her finesse extends to different aspects of the healthcare provider/consumer relationship including, but not limited to, health management, compliance, reactions to promotional activties and the buying process of over the counter products.
Rose-Marie Carneiro
Business Unit Director
Patient Research
Sigi started his career 1989 at Novartis pharma where he worked in different functions of market research on national and European level. This included covering a wide range of aspects related to quantitative and qualitative market research, analytics, strategic planning and forecasting. Later he joined TNS/Kantar Health, working on mainly large scale international quantitative studies. He gained a deep understanding of qualitative projects during his first years in London. Over the years, Sigi worked on multiple therapy areas and brands, ranging from CV/CNS to immunology, oncology, rare diseases and biosimilars.
Sigi joined APLUSA in 2019 after contracting roles for BMS and GSK. He is based in the London office of AplusA.
Siegfried Ertl
International Business Unit Director Syndicated Research Solutions
Stéphane joined APLUSA with over 20 years of experience in clinical trial management and business development within Pharma and CRO sectors. With customer satisfaction as a leitmotif, he excels at providing tailored full-service solutions, including Clinical, Data Science, RWE, in areas such as Oncology, Cardiology, CNS, Metabolism, and Rare Diseases. His efforts span multiple applications in Europe, the US, and China, and include initiating a Real World Evidence Business Line to improve market access and patient outcomes. Stéphane holds a Master’s degree in Biology and an engineering degree in fundamental research from an academic institution, blending scientific expertise with a strategic sales approach to focus on client needs and return on investment through robust, practical solutions.
Stéphane DENIAU
Business Unit Director
APLUSA REAL WORLD
Susana has over 17 years of experience in the Pharmaceutical Industry. She began her career at Boehringer Ingelheim as Brand Manager, then as Account Director at Medimix Europe, before joining APLUSA as research Director in 2016. She successfully conducts large global quantitative and qualitative international ad hoc studies for top European and American pharmaceutical companies. Susana graduated in Business Administration from UCAB in Venezuela and a Master in Marketing Management from the University of Westminster (UK).
Susana Suarez
International Business Unit Director
William joined APLUSA after over 10 years spent as an Account Director in IFOP’s Healthcare department. William has cumulated 30 years of experience working in the market research industry and over 13 years solely in Healthcare. William brings in solid professional skills in consumer behavior, customer insight, qualitative and quantitative research, with a strong focus on in-depth understanding of market trends and delivery of smart, operational findings.
William MacGillivray
International Business Unit Manager
Business Development and business unit Directors
Eric has joined APLUSA after over 20 years dedicated at growing exceptional relationships with clients in the pharmaceutical industry across Europe and Asia. Eric brings in a solid expertise in the sale of services (market research studies, databases, panels of physicians and pharmacies) to help implementing, together with our Business Unit Directors and experts, commercial partnerships that will match even more our clients’ expectations. Graduate of the Ecole Supérieure de Gestion (now Paris School of Business), Eric also holds a master’s degree in economic and social administration from the University of Paris-Assas and an Executive MBA in healthcare management from the University of Paris-Dauphine.
Eric Yonter
Business Development Director
Elen joined APLUSA in 2005 and has over 15 years of experience in Healthcare Market research. Moving from Project Manager to Research Director positions, she conducted qualitative and quantitative research, with more focus on respiratory diseases, rheumatology, ophthalmology, dermatology, neurology, diabetes, medical devices, and Women’s health. Elen holds a Master degree in Marketing and Business management performed in France, Canada and the UK.
Elen Treuveur
France Business Unit Director
Fabrice has over 15 years of experience in healthcare Market Research. His first position was at APLUSA in Lyon from 2005 to 2008 before holding various senior positions in other international healthcare market research companies in Switzerland, the UK, and the US where he has settled permanently, before returning to APLUSA in 2023. Fabrice is passionate about innovative research methodologies uncovering human truths to inform brand development strategies. Fabrice also loves transforming insights into clear, simple, and actionable business solutions. Fabrice is a specialist in several therapy areas, in particular Immunology and HIV. Fabrice holds a Master’s degree in chemical process engineering, followed by an MBA in Pharmaceutical Marketing and Management from ESCP Business School.
Fabrice Dussol
US Business Unit Director
With 15 years of experience in market research and strategic marketing (mainly at Publicis Healthcare), Geoffroy Cornette has joined forces with the team at AplusA to accompany clients from initial consultation to project completion, providing critical strategic planning support. Geoffroy uses his expertise and passion in prescription markets to help you to map patient journeys, uncover prescription drivers, optimize communication, …, with a focus in oncology, hematology, virology, respiratory disease, and dermatology. Geoffroy graduated from EDC Paris Business School and IDRAC Business School.
Geoffroy Cornette
France Business Unit Manager
Hilary has over 25 years of experience in healthcare, having begun her career in the pharma industry before moving agency-side. She specializes in international/global studies, both qualitative and quantitative, and is passionate about the application of market research insights to support business decision making, as well as advocating for the voice of the patient in market research. Hilary is a specialist in several therapy areas, in particular multiple sclerosis and oncology, and has a literature degree from Leicester University as well as postgraduate marketing qualifications.
Hilary Worton
International Business Unit Director
Justin started his career at APLUSA in 2014 as a Market Research Executive, specializing in prescription analysis and ATU studies. Elevating swiftly, he assumed a leadership role as a Project Manager in the French Quant Team, overseeing comprehensive quantitative research across various stages of the product lifecycle. In 2021, Justin transitioned to the international team, where he currently serves as Business Unit Manager, showcasing profound expertise in Hematology (DLBCL, CLL, ALL, Multiple Myeloma), Oncology (Breast, Lung, Ovarian, Prostate), and Immunology. Justin holds a master’s degree in Marketing and Strategy.
Justin François
International Business Unit Manager
After first working in consumer research, Magali joined APLUSA in 2002 and specializes in patient chart studies covering all stages of the product lifecycle.
Through her expertise in a wide range of disease areas (hematology, oncology, infectious diseases, dermatology, CNS). Magali graduated in Psychology and holds a Master’s degree in Market Research, Marketing and Communication (Sciences Po Grenoble).
Magali Marion
France Business Unit Director, Prescription
Manuel has over 17 years of experience in the pharmaceutical and MedTech market research industry. He began his career at APLUSA where he went through different positions until research director, managing large international projects during 10 years in different disease areas including oncology, hematology, cardiology, respiratory, diabetes, ophthalmology, aesthetics, infectious diseases, etc. In 2015 he joined bioMérieux as Global Market Research Manager where he supported marketing teams over 7 years to collect the voice of the customer to inform decision making for their major brands in the fields of microbiology, molecular biology and immunoassay. He also provided support in other strategic projects related to the evaluation of new portfolio opportunities. Manuel joined back APLUSA as Business Unit Director. Manuel graduated in Business Administration in the University of Granada (Spain) and complemented it with a master’s in international trade and European affairs from IAE Lyon.
Manuel Guzman Martin
International Business Unit Director
Specializing in French domestic market research studies, Marie-Christine Ducrot serves as a Business Unit Director. She has a particular interest in market research involving psychological aspects of physicians’ decisions including relationships between patients/physicians and physicians/pharma companies as well as the evolution of the role of HCPs and the global impact. Marie Christine holds a DEA in Experimental and Social Psychology
Marie Christine Ducrot
France Business Unit Director
Mélanie joined APLUSA in 2009. She has developed tremendous expertise in prescription research over the years, holding the role of Director of the production team, bringing in her specific skills of past scientific researcher in CNRS (the French National Centre for Scientific Research, among the world’s leading research institutions). She is now helping you to design and conduct any type of market research based on patient charts, with experience in numerous domains such as oncology, immune-oncology, rheumatology, dermatology, gastro-enterology, hematology, ophthalmology, cardiology, etc. Mélanie holds a PhD in Biology and Chemicals.
Mélanie Marrot, PhD
France Business Unit Director, Prescription
Nadège joined APLUSA in 2020 to develop international market research in OTC medicines and products.
She has a strong background in marketing and product management (conditions: anxiety, stress, baby teething, naupathia… / products: dietary supplements, medicines, cosmetics, medical devices). Nadège started her career agency side as a quantitative market research executive before working client side in OTC products for a pharmaceutical company.
Her roles have included data research executive, then international product manager and head of market research and data panels. Nadège is a marketing graduate (Toulouse Business School), she speaks Italian, French and English.
Nadège Meillier
International Business Unit Director OTC & Consumer Healthcare
Pascale has over 20 years of experience in Healthcare market research and joined APLUSA in 2006. Psycho-sociologist by training, Pascale has developed expertise in qualitative and quantitative techniques and is particularly interested in research looking at all environment around patient with chronic or rare diseases (patient journey, interactions with caregivers and HCPs). She is also expert in OTC and consumer healthcare, nutrition, skin care, pediatrics, and rheumatology. Pascale holds a Master degree in Psychology and Sociology with specialization in consulting.
Pascale Bausson
France Business Unit Manager
Paul has been working in international healthcare market research for over 20 years, and is based in our London office. Paul is responsible for supporting global and UK-based clients with their needs across all therapy areas and methodologies. Paul enjoys studies that include patients, as well as those relating to medical devices, and spent numerous years working with longitudinal data from hospital records in Europe. Paul has previously worked with Aequus Research, Medimix and IQVIA, successfully developing new business among pharmaceutical and Biotech clients in Europe, Japan, Latin America and the USA. Paul has a wealth of experience within various therapeutic classes, noticeably in Oncology and Multiple Sclerosis as well as Diabetes, Epilepsy, HIV, pain management and Anti-Infectives.
Paul Taylor
International Business Unit Director
Rose-Marie Carneiro is a Business Unit Director with more than 20 years’ experience in pharmaceutical market research in both France and Australia.
As an expert in both qualitative and quantitative market research techniques, Rose-Marie pays special attention to patients and consumers in different therapeutic areas such as CV, respiratory, CNS, diabetes, aesthetics and cosmetics, women’s health, baby nutrition, senior nutrition, pain management and wellbeing. On top of this, her finesse extends to different aspects of the healthcare provider/consumer relationship including, but not limited to, health management, compliance, reactions to promotional activties and the buying process of over the counter products.
Rose-Marie Carneiro
Business Unit Director
Patient Research
Sigi started his career 1989 at Novartis pharma where he worked in different functions of market research on national and European level. This included covering a wide range of aspects related to quantitative and qualitative market research, analytics, strategic planning and forecasting. Later he joined TNS/Kantar Health, working on mainly large scale international quantitative studies. He gained a deep understanding of qualitative projects during his first years in London. Over the years, Sigi worked on multiple therapy areas and brands, ranging from CV/CNS to immunology, oncology, rare diseases and biosimilars.
Sigi joined APLUSA in 2019 after contracting roles for BMS and GSK. He is based in the London office of AplusA.
Siegfried Ertl
International Business Unit Director Syndicated Research Solutions
Stéphane joined APLUSA with over 20 years of experience in clinical trial management and business development within Pharma and CRO sectors. With customer satisfaction as a leitmotif, he excels at providing tailored full-service solutions, including Clinical, Data Science, RWE, in areas such as Oncology, Cardiology, CNS, Metabolism, and Rare Diseases. His efforts span multiple applications in Europe, the US, and China, and include initiating a Real World Evidence Business Line to improve market access and patient outcomes. Stéphane holds a Master’s degree in Biology and an engineering degree in fundamental research from an academic institution, blending scientific expertise with a strategic sales approach to focus on client needs and return on investment through robust, practical solutions.
Stéphane DENIAU
Business Unit Director
APLUSA REAL WORLD
Susana has over 17 years of experience in the Pharmaceutical Industry. She began her career at Boehringer Ingelheim as Brand Manager, then as Account Director at Medimix Europe, before joining APLUSA as research Director in 2016. She successfully conducts large global quantitative and qualitative international ad hoc studies for top European and American pharmaceutical companies. Susana graduated in Business Administration from UCAB in Venezuela and a Master in Marketing Management from the University of Westminster (UK).
Susana Suarez
International Business Unit Director
William joined APLUSA after over 10 years spent as an Account Director in IFOP’s Healthcare department. William has cumulated 30 years of experience working in the market research industry and over 13 years solely in Healthcare. William brings in solid professional skills in consumer behavior, customer insight, qualitative and quantitative research, with a strong focus on in-depth understanding of market trends and delivery of smart, operational findings.
William MacGillivray
International Business Unit Manager
Qualitative Expert CONSULTANT
Anna Robbertz has over 25 years of consulting and marketing research experience as well as another five years in pharmaceutical marketing on the client side. Anna has extensive experience across a range of therapeutic areas. Anna has expertise in both qualitative and quantitative marketing research. She is best known for qualitative research and is highly-sought as a moderator. Anna’s research skills include in-depth, one-on-one interviewing and focus group moderation for a range of stakeholders, including HCPs, KOLs, patients, sales reps, and payers. Anna’s skills touch on all areas of qualitative research, including discussion guide development, interviewing, data analysis, report writing, and presentation of findings. She has extensive experience in managing global qualitative projects. Anna has an MBA in Marketing from Bentley College, and a B.S. in Public Health from the University of Massachusetts at Amherst. Anna is a native of the Netherlands.
Anna Robbertz
US Qualitative Expert Consultant
Qualitative Expert CONSULTANT
Anna Robbertz has over 25 years of consulting and marketing research experience as well as another five years in pharmaceutical marketing on the client side. Anna has extensive experience across a range of therapeutic areas. Anna has expertise in both qualitative and quantitative marketing research. She is best known for qualitative research and is highly-sought as a moderator. Anna’s research skills include in-depth, one-on-one interviewing and focus group moderation for a range of stakeholders, including HCPs, KOLs, patients, sales reps, and payers. Anna’s skills touch on all areas of qualitative research, including discussion guide development, interviewing, data analysis, report writing, and presentation of findings. She has extensive experience in managing global qualitative projects. Anna has an MBA in Marketing from Bentley College, and a B.S. in Public Health from the University of Massachusetts at Amherst. Anna is a native of the Netherlands.
Anna Robbertz
US Qualitative Expert Consultant
Quality and Compliance
APLUSA has obtained ISO 20252-2019 certification: “Market and opinion research, data analysis, strategic and operational consulting for global healthcare markets, in compliance with applicable regulations, in order to improve the reliability of our clients’ decisions and to contribute to better patient care.”
We comply with industry best practices and recognize the importance of a stringent but fluid compliance to international and local regulations. Our process is continuously adjusted according to the most up-to-date legal and ethical requirements; and staff members receive the necessary training under the supervision of the quality management team.

Quality
Process, Training, Control
- Quality Department
- APLUSA’s Standard process
- Quality Management System
- Client Satisfaction
- Continuous improvement

Pharmacovigilance
Adverse Event Reporting
- APLUSA’s AER process frequently updated
- 100% internal staff training
- 100% fieldwork partners training

Safety
Data protection, Data preservation
- Secured access to data
- Confidentiality clause
- Business continuity plan
- Backup and restore process
Our corporate Social Responsibility (CSR)
APLUSA’s Commitment to Sustainability
We are proud to share our first Sustainability Report, detailing our environmental, social, and governance (ESG) initiatives and achievements. This report highlights APLUSA’s dedication to positively impacting society and the environment, while maintaining strong economic foundations. By publishing our goals, actions, and measurable outcomes, APLUSA aims to foster transparency and accountability in our journey towards sustainable development, creating shared value for our stakeholders and communities alike.
Discover our Sustainability Report here 👉
APLUSA has been awarded since 2021 silver medal to assess superior APLUSA’s sustainability performance in our industry, which goes beyond compliance.
This rating covers a broad range of management systems including Environmental, Labor & Human Rights, Ethics, and Sustainable Procurement impacts.

Index for professional equity between women and men
French regulation makes it mandatory to publish the “index for professional equity” between women and men in the French subsidiary. This index is 92/100 for APLUSA in 2023 and reflects how much aware is APLUSA of the importance of professional diversity, and considers it as a factor of collective enrichment, social balance, and economic efficiency.
APLUSA fights against all forms of discrimination and promotes gender parity in order to guarantee equality of consideration to all aplusians. APLUSA scores 92/100 in this official Professional Equality Index between women and men published in 2024 for the year 2023.
APLUSA supports this process of inclusion, and diversity, and is determined to eliminate any identified gaps. Day after day, APLUSA places gender parity at the heart of its values.
Let’s talk!
Want to find out more information about APLUSA?
Whether you would like to discuss a specific project or hear more about our services and expertise – we’d be delighted to hear from you.